
To date, no significant side effects have been reported in the interim phase 3 studies of the Moderna and Pfizer vaccines. No mRNA vaccines have been approved before because it is relatively new technology.īut these mRNA vaccines appear safe and no riskier than other tried and tested ones, like the childhood measles vaccine. The upside is that if the vaccine is safe and effective, it can be distributed immediately, and vaccination can begin. To this end, the pharmaceutical companies launched at-risk manufacturing – which means that the manufactured vaccine doses would be thrown away if the vaccine was ineffective or unsafe – during the FDA-mandated two-month safety waiting period. government wanted to be ready to begin distributing the vaccine the moment the results of the phase 3 trials were known and the safety data had been analyzed. Typically, large-scale manufacturing begins only once the vaccine has been tested in clinical trials. The reason that vaccines may be approved so quickly is that the large clinical trials to assess vaccine efficacy and safety are happening at the same time as the large-scale manufacturing preparation, funded by the federal government’s Operation Warp Speed program. In my opinion, safety is not compromised by the speed of vaccine development and emergency use authorization. Safety is the first and foremost goal for a vaccine. How is safety assured when vaccine development is so fast? Manufacturing an mRNA vaccine rather than a protein vaccine can save months, if not years.Īnother factor that accelerated vaccine development was the swift and efficient recruitment of patients for clinical trials. The genetic material mRNA is easy to make in a laboratory.
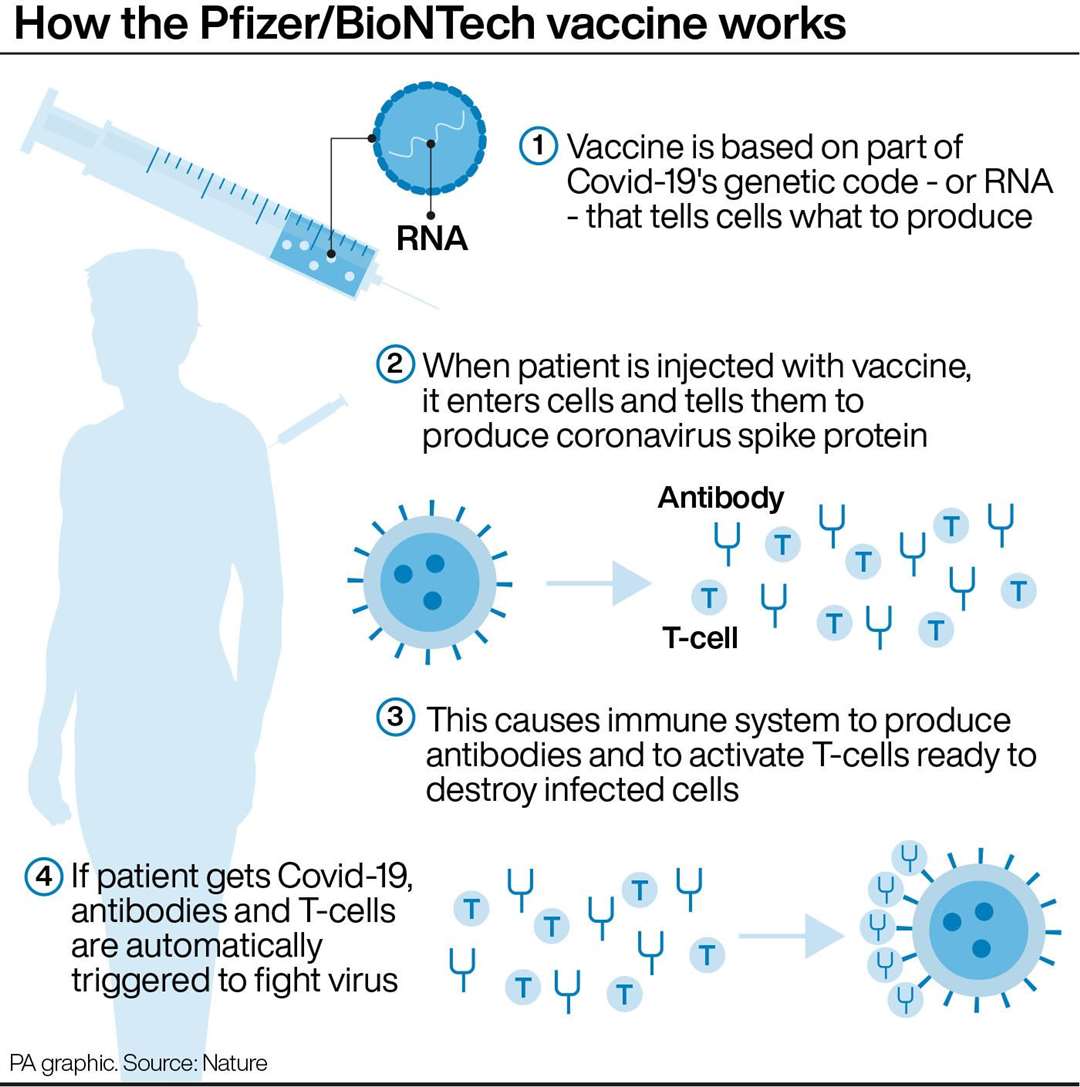
By contrast, by using just the genetic material that makes the Spike glycoprotein – the protein on the surface of the coronavirus that is essential for infecting human cells – the design and manufacture of the vaccine is simplified. Traditional vaccines typically use a weakened version of the pathogen or a protein piece of it, but because these are grown in eggs or cells, developing and manufacturing vaccines takes a long time. The spike proteins (red) on the surface of SARS-CoV-2, the virus that causes COVID-19, are essential for infecting human cells. It took Jonas Salk six years to develop and test the first polio vaccine, starting with the isolation of the virus.
It also took 15 years to develop a vaccine for rotavirus, which commonly causes severe, watery diarrhea. It took 15 years to develop a vaccine for human papilloma virus, which can cause six kinds of cancer. Both the chickenpox vaccine and FluMist, which protects against several strains of the influenza virus, took 28 years to develop. Vaccines typically take at least a decade to develop, test and manufacture. How long does most vaccine development take? I’m also conducting research on how dysregulation of the immune system during SARS-CoV-2 infection causes lung damage.ĭespite the vaccines’ relatively rapid development, the normal safety testing protocols are still in place.
#Moderna and pfizer vaccine production trial
I care for patients with COVID-19 and am conducting the local site for a phase 3 clinical trial of Regeneron’s antibody cocktail as a tool to prevent household transmission of COVID-19. I am an infectious diseases specialist and professor at the University of Virginia. Both were developed in a record-breaking 11 months or so.
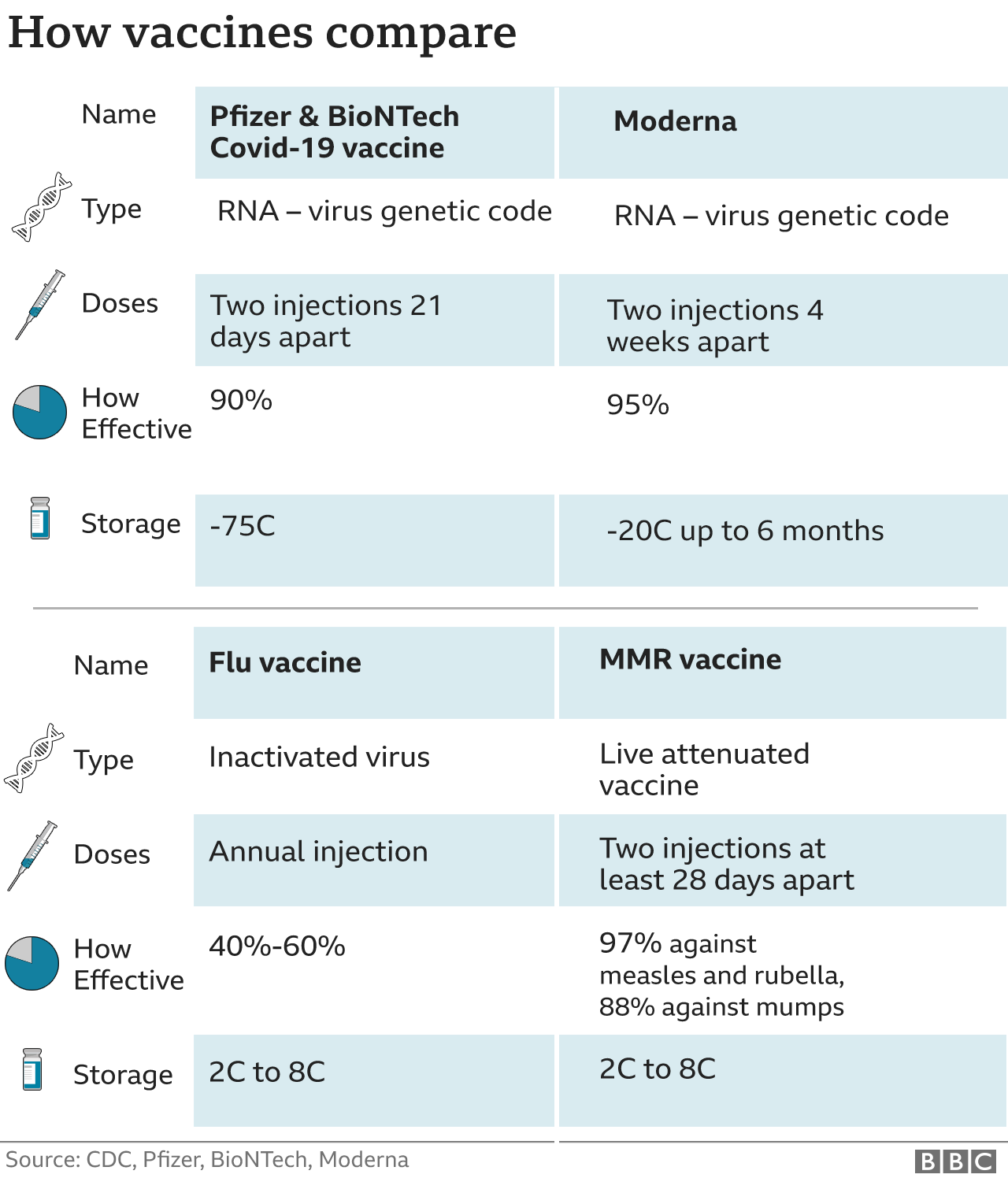
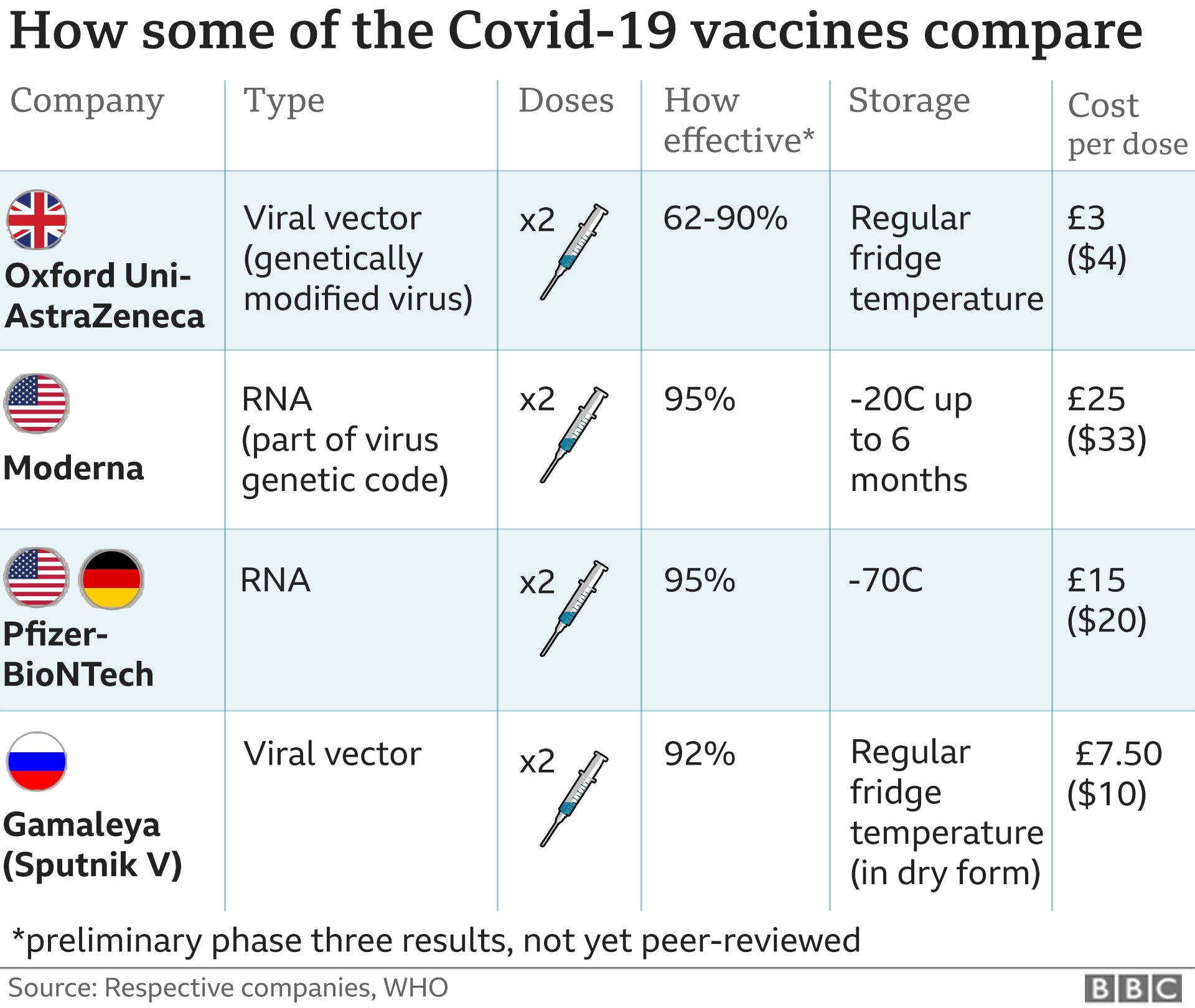
There are now two COVID-19 vaccines that, at least according to preliminary reports, appear to be 94.5% and 95% effective.


 0 kommentar(er)
0 kommentar(er)
